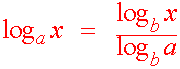Log Bases and Log equations
The Common Logarithm
In chemistry, base 10 is the most important base. We write
to mean the log base ten of x.
Example:
log 10,000,000 = log 107 = 7
and
log 0.00000001 = log 10-8
Example
We can see that
log 12,343,245
is between 7 and 8 since
10,000,000 < 12,343,245 < 100,000,000
log 10,000,000 = 7
and
log 100,000,000 = 8
Example
We can see that
log 0.0000145
is between -5 and -4 since
0.00001 < 0.0000145 < 0.0001
and
log 0.00001 = -5
and
log 0.0001 = -4
Exercise
Use your calculator to find
log 1,234
![]()
and
log 0.00234
![]()
Change of base formula
We next want to be able to use our calculator to evaluate a logarithm of any base. Since our calculator can only evaluate bases e and 10, we want to be able to change the base to one of these when needed. The formula below is what we need to accomplish this task.
|
Change of Base Formula
|
Proof
We write
y = loga x
So that
ay = x
Take logb of both sides we get
logb ay = logb x
Using the power rule:
y logb a = logb x
Dividing by logb a
logb
x
y =
logb a
Example
Find
log2 7
We have
log 7
log2 7 =
= 2.807...
log 2
Log Equations
Example
Solve
log2 x - log2 (x - 2) - 3 = 0
We use the following step by step procedure:
Step 1: bring all the logs on the same side of the equation and everything else on the other side.
log2
x - log2(x - 2) = 3
Step 2: Use the log rules to contract to one log
x
log2
= 3
x - 2
Step 3: Exponentiate to cancel the log (run the hook).
x
= 23 = 8
x - 2
Step 4: Solve for x
x = 8(x - 2) = 8x - 16
7x = 16
16
x =
7
Step 5: Check your answer
log2 (16/7) - log2 (16/7 - 2) = 3
Exercises:
log(x + 2) - log(x - 1) = 1

log2(x) + log2(x + 5) = 2

Exponential Equations
Example
Solve for x in
2x - 1 = 3x + 1
Step 1: Take logs of both sides using one of the given bases
log2 2x - 1 =
log2 3x +
1
Step 2: Use the log rules to simplify
x - 1 =
(x + 1) log2 3 = (x + 1)(log 3)/(log2) =
1.58(x
+ 1)
Step 3: Solve for x
x - 1 = 1.58 x + 1.58
-.58 x = 2.58
x =
-4.45
Step 4: Check your answer.
Exercises
-
3x - 2 = 52x - 3

-
21 - x = 3x - 1

For an interactive computer lesson on exponential equations click here
or to play the log memory game click here
Back to the Exponentials and Logs Home Page
Back to the Intermediate Algebra (Math 154) Home Page
Back to the Math Department Home Page
e-mail Questions and Suggestions